-
CTMS integration info
ClinCapture integrates seamlessly with Clinical Trial Management Systems (CTMS), allowing customers to use our innovative Electronic Data Capture (EDC) in conjunction with CTMS for maximum convenience. These integrations allow customers to achieve one-stop-shopping with ClinCapture for EDC and CTMS solutions which can lower costs, accelerate timelines, and allow Data Managers, Monitors, and Clinical Research Assistants (CRAs) to work more efficiently together.
-
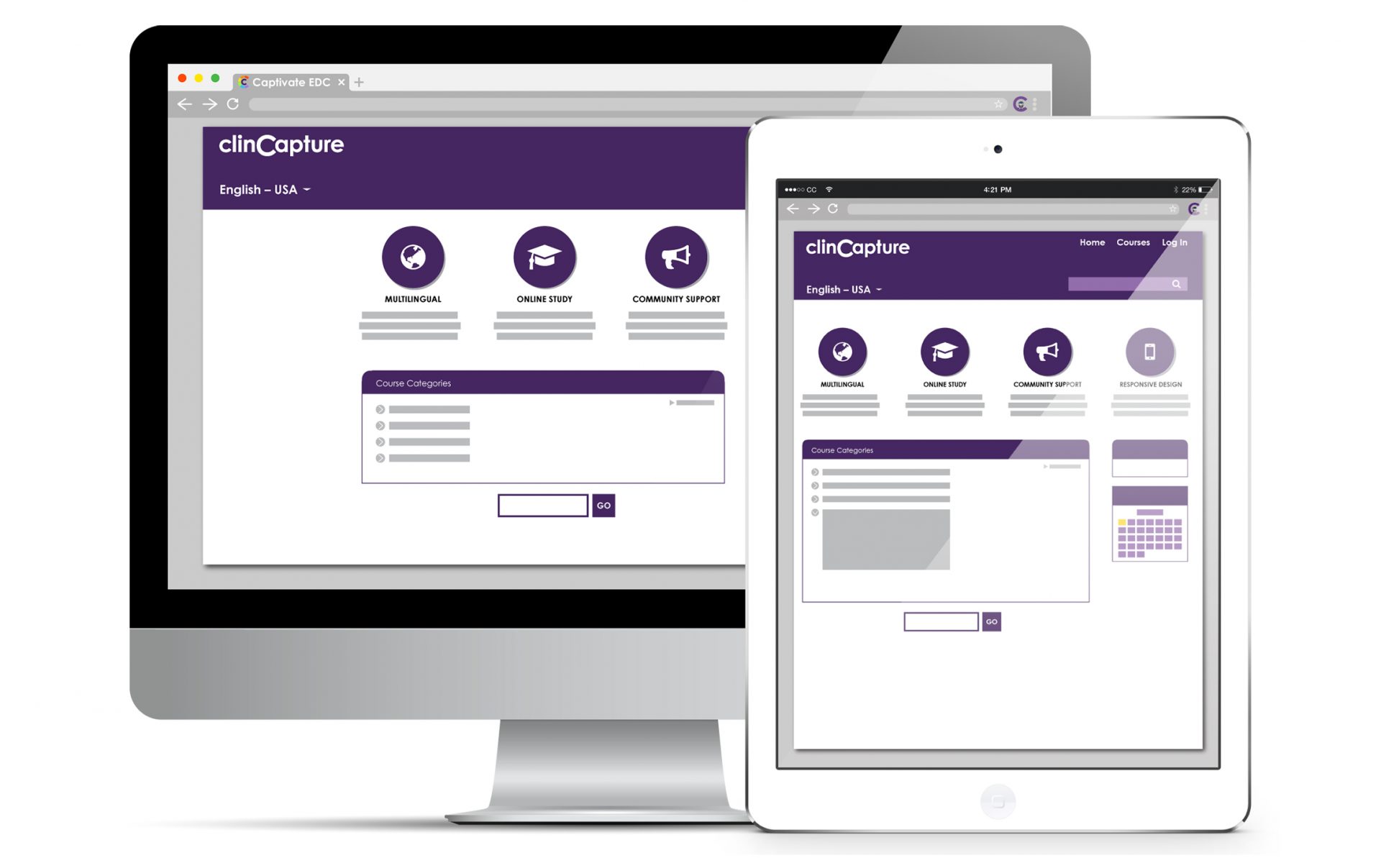
Learning Management System (LMS)
With ClinCapture ’s Learning Management System (LMS) integration, users can learn how to use Captivate® at their own pace. This interactive training is divided into modules and users have the opportunity to take a quiz and to get certified at the end of their training. Users can access general or role-specific training.
-
API and Clinical Views
Captivate® EDC allows user accounts to access web service APIs for external integrations, including CTMS, Safety, and Lab Systems. Captivate® can import data from external web-based services (Example: JSON/XML). This eases workflow and eliminates the need for manual entry.
In addition, Clinical Data Views provide access to a normalized view of the clinical data for reporting and business intelligence (BI) tools. Any BI tool supporting PostgreSQL can be used.